Abstract
Introduction: Although end of treatment (EOT) response assessment using Fluorine-18-fluorodeoxyglucose positron emission tomography (PET) has proven highly predictive of disease remission in Hodgkin lymphoma (HL) and aggressive non-Hodgkin lymphoma, there is little data on the prognostic significance of PET response in patients with post-transplant lymphoproliferative disorder (PTLD). Optimal treatment of patients with PTLD is complex and better understanding of the significance of EOT imaging could help guide subsequent therapeutic decisions.
Methods: As part of a multi-institutional study of viral genomes in PTLD, we collected clinical data (demographics, transplant and PTLD characteristics, treatment, imaging, and patient survival) from three large organ transplant centers in the United States from 1991 onwards. Patients who were PET negative at EOT considered to have a complete remission (CR), patients with decrease in FDG avidity who were still PET positive considered to have a partial remission (PR), and patients with stable or increasing FDG avidity considered to have no remission or progressive disease (NR/PD). Time-to-event outcomes (time to death and time to death or relapse) were analyzed using the Kaplan Meier method.
Results: Among 147 pediatric and adult patients with PTLD, imaging at diagnosis and EOT included PET n=94 (64%), computed tomography (CT) n=77 (53%), and/or magnetic resonance imaging (MRI) n=14 (10%). Median recipient age at first transplant was 29.6 years (IQR 14.8 to 25.9), and 57.5% were male. Transplants included kidney (29%), heart (27%), liver (18%), lung (18%), hematopoietic (7%), intestine (6%), or pancreas (3%). Sites of PTLD were lymph node (57%), gastrointestinal tract (34%), lung (19%), disseminated (10%), liver (8%), central nervous system (4%), kidney (3%), bone marrow (3%), or other (41%). PTLD subtypes included monomorphic diffuse large B-cell lymphoma (DLBCL) (69%), polymorphic (17%), classical HL (8%), or non-destructive (5%). All non-destructive and polymorphic PLTD was Epstein-Barr virus (EBV) positive while 60.6% of DLBCL and 75% of classical HL were EBV positive. Treatments varied but included reduction of immunosuppression in 65.6% and rituximab or chemotherapy in 62.4% of patients.
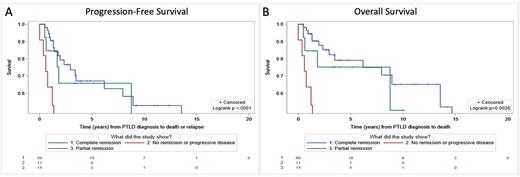
For 84 patients with PET scans and outcome data, patients who achieved CR (59/84; 70.2%) experienced a subsequent progression-free median survival of 13.5 years compared with 8.8 years for patients with PR (11/84; 13.1%) and 1.4 years for patients with NR/PD (14/84; 16.7%) (p<0.0001, Figure A). Median overall survival was 14.7 years for patients with CR (60/85; 70.6%), not reached for patients with PR (11/85; 12.9%), and 1.4 years for patients with NR/PD (14/85; 16.5%) (p=0.0026, Figure B). 20.8% of patients who achieved CR subsequently relapsed compared to 42.9% of patients with PR. 81.8% of patients with NR/PD experienced relapse or death.
Conclusion: End of therapy PET identifies a subgroup of patients with CR who are at low risk for relapse. While patients with NR/PD had poor prognosis, patients with PR represent an intermediate group who are PET positive at the EOT but can achieve long-term remission without additional therapy.
Disclosures
Marks:Abbvie: Honoraria. Green:LTB-Med: Consultancy; Allovir: Consultancy; Bristol Myers Squibb: Consultancy. Dharnidharka:Atara Bio: Consultancy; CareDx: Honoraria, Research Funding; Transplant Genomics: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.